estimation of barium as barium sulphate by gravimetric method|barium baso4 ppt : distribute The Gravimetric Estimation of Barium: The given barium chloride solution is made up to a definite volume. A measured volume of it is then treated with dilute sulphuric .
12 de jan. de 2024 · Bimi Sushi, #1 entre Guarulhos restaurantes de sushi: 7065 avaliações e 166 fotos detalhadas. Encontrar no mapa e ligar para reservar uma mesa.
{plog:ftitle_list}
web7 de ago. de 2023 · Renata Frisson, a Mulher Melão, surpreendeu os fãs com uma live na qual aparece pelada. A influenciadora disponibilizou o conteúdo, gravado em um hotel de luxo no Rio de Janeiro, para os fãs em uma plataforma adulta. “Eles foram à loucura. Live é a parte do meu canal que eles mais amam, porque interajo com eles e fico bem à vontade.
The document describes a procedure to estimate the amount of barium in a sample of barium sulfate using gravimetric analysis. An unknown sulfate salt is dissolved and reacted with barium chloride, precipitating out barium sulfate. . The barium sulfate precipitate is collected by filtration, dried and weighed. Since barium chloride is added in excess, and since the precipitation reaction goes to completion, we . This experiment aims to determine the amount of barium present in a solution by precipitating it as barium sulfate and measuring the mass of the solid produced. The apparatus needed includes a watch glass, beaker, funnel, .
In this video we had discussed about Estimation of Barium Sulphate by Gravimetric Analysis 1. Principle of Estimation of Barium Sulphate by Gravimetric Analysis .Using this method allows you to add the barium chloride solution to the beakers while they are continuing to be heated on the hot pads. The filtration will be carried out using glass or plastic . The Gravimetric Estimation of Barium: The given barium chloride solution is made up to a definite volume. A measured volume of it is then treated with dilute sulphuric .By EVELYN M. COCHRAN, ERVIN B. INSKIP, PERRY KING, and HOWARD W. ZIEGLER. A method suitable for compendia1 assay of barium sulfate USP has been developed. Barium is .
In this experiment you will determine the percentage (by mass) of sulphate in an unknown sulphate salt by gravimetric analysis. Sulphate salt is precipitated by barium chloride as .
barium to baso4
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.Gravimetric Analysis of SULFATE as Barium Sulfate. Objectives • • To learn the techniques associated with gravimetric analysis. . Background Gravimetric analysis is a quantitative method for accurately determining the amount of a .Turbidimetric method. 1. Gravimetric Method with Ignition of Residue Principle Sulphate is precipitated in hydrochloric acid medium as barium sulphates by the addition of barium chloride. The precipitation is carried out near the boiling temperature and after a period of digestion the precipitate is filtered; washed with water until free of .
ADVERTISEMENTS: In this article we will discuss about the gravimetric method for estimation of sulphate in plants. Principle: Sulphate is precipitated as barium sulphate in hydrochloric acid medium by the addition of barium chloride solution. The reaction is carried out near the boiling temperature. The precipitate is filtered and washed to remove the chlorides, then [.]
barium baso4 ppt
The estimation of barium sulfate by gravimetric analysis involves the precipitation of barium ions as barium sulfate, followed by the separation, washing, and weighing of the precipitate. . Finally, a method using barium sulfate precipitation and EDTA volumetric titration can be used to measure the barium content in barium aluminosilicate and . The method is based on the turbidimetric determination of sulphate using barium chloride as reagent and measuring the absorbance of the formed suspension spectrophotometrically at 540 nm.
1.2 This method is suitable for all concentration ranges of sulfate (SO 4-2); however, in order to obtain reliable readings, use a sample aliquot containing not more than 40 mg/L of SO 4-2. 1.3 The minimum detectable limit is approximately 1 mg/L of SO 4-2. 2.0 SUMMARY OF METHOD 2.1 Sulfate ion is converted to a barium sulfate suspension under
Gravimetric Analysis as one of the Analytical method for the Quantitative Determination of sulphate ions from aqueous media being the most simplest, rapid and low-cost method. There are 2 gravimetric methods, namely : precipitation and eva poration method. In gravimetry we must pay attention to accuracy in work, because what is need is a quantita tive
4) Suppose that a small portion of the sulfate precipitated as lead sulfate rather than as barium sulfate. How this would change the result of the analysis? 5) From the following list, identify the interfering species in the sulfate determination method used in this experiment: Pb2+, Na+, NO 3-, CO 3 2-, PO 4 3-.Gravimetric analysis is a quantitative evaluation of laboratory techniques based primarily on the size of an analyte’s mass. Gravimetric analysis can be used in real life for many users, such as monitoring lead levels in water for human consumption, which, if not monitored, could lead to poisoning and death.estimation of barium as barium sulphate || estimation of barium sulphate by gravimetric analysis #sgin this video we cover 1. estimation of baso42. estimatio.
aroya solus teros 12
Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed . • Purity of the precipitate: co-precipitation and post precipitation, • Estimation of barium sulphate 11/1/2018 Deokate U A 2 3. . • Gravimetric method is one in which the analysis is completed by a weighing operation. • Gravimetric Analysis is a group of analytical methods in which the amount of analyte is determined by the .
The Gravimetric Estimation of Barium: The given barium chloride solution exists made up of a definite volume. A measured volume of it exists then treated with dilute sulphuric acid and barium precipitated as barium sulphate. The precipitated barium sulphate exists split and weighed. The mass of Barium in the total of the given solution exists .What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a .Estimation of Barium from Barium Sulphate Gravimetrically Objectives The objectives of this laboratory are as follows: • To experimentally analyze an unknown sulfate salt via a precipitation reaction, using the techniques .
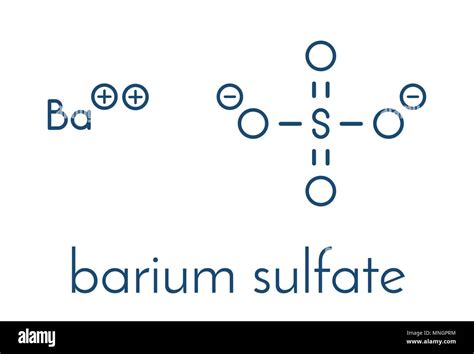
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as .Sulphate Gravimetric Method for Sulphate Determination: Theory: Nearly all sulphates in water can be precipitated as BaSO 4 on reaction with barium chloride (BaCl 2) under acidic conditions. Since this compound has very low solubility product, the . 8B Estimation of Sulphate in Water A. Lab Report: (4 x 10 Marks = 40 Marks)What is Barium Sulphate (BaSO 4)? BaSO 4 is an inorganic compound with the chemical name Barium Sulphate. Barium Sulphate is composed of a barium cation and the sulphate anion. The sulphur is attached to four oxygen atoms. BaSO 4 is a sulphate salt of barium and is found as the mineral bariteCalculation: Mass of crucible + lid =(a)g Mass of crucible - lid + Barium sulfate (b) Mass of Barium sulfate = (b-a) - 233.36g of barium sulfate contain 137.36 g of barium Mass of barium in (b-a)g of Barium sulfate = Therefore, Mass of Barium in the whole of the given solution = Result: Mass of Barium in the whole of the given solution
michell instruments humidity calculator
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Barium sulfate frequently acts as a tracer or marker in laboratory experiments and may be present in environmental samples, emphasizing the significance of accurately determining its presence. One of the most common methods for estimating barium sulfate is gravimetric analysis, which relies on the precipitation of barium sulfate as a solid residue. Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . When all sulphate ions have precipitated as barium sulohate (BaSO 4), the precipitate of barium sulohate is filtered . This method has been successfully used to estimate a wider range of organic compounds . The objective of the experiment is to determine the amount of sulphate by the gravimetric method. In this experiment, the precipitation gravimetric method is used. The unknown sulfate solution is converted into an insoluble white compound, BaSO 4. It is then filtered by suction filtration, washed, dried and weighed to determine the amount of .
#inhindi gravimetric estimation of barium sulphate || estimation of barium as barium sulphate by gravimetric method || describe gravimetric determination of .into a suspension of barium sulphate which is then determined turbidimetrically. You would be using a spectrophotometer to determine the turbidity of a solution. When carefully performed, the turbidimetric method provides reproducible results for such an important determination. This method is much faster and sensitive as compared to thefor the determination of sulfate. Which indication method is the most suitable depends above all on the sample matrix. Method 1: Precipitation as barium sulfate and back-titration of the Ba2+ excess with EGTA. The ion-selective calcium electrode is used as indicator electrode. Method 2: As in method 1, but with the electrode combination .

Para fazer o download do CopySpider 2.3.0 (23/01/2024), faç.
estimation of barium as barium sulphate by gravimetric method|barium baso4 ppt